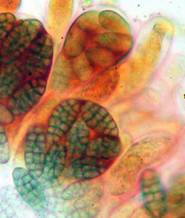
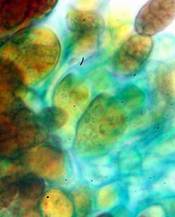
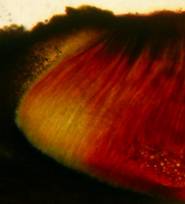
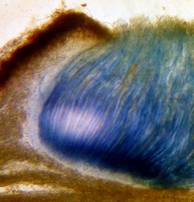
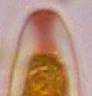
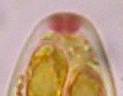
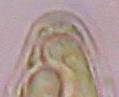
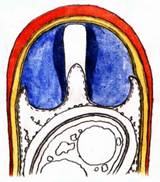
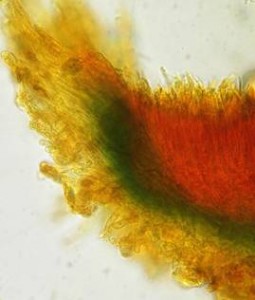
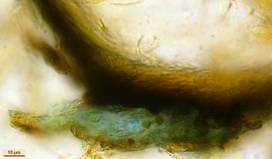
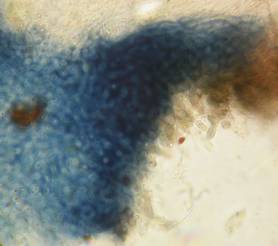
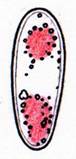
Hans-Otto Baral, Jan. 2009
Abstract. In mycology the terms amyloid and amyloidity are currently applied to a blue iodine reaction of fungal microstructures. This colour reaction is explained by an iodine complex with helicoid carbohydrate macromolecules. It is mainly observed when adding iodine reagents to ascus walls and ascus apical rings, but also to basidiospore walls and other fungal structures. Amyloidity is used as a very important characteristic in fungal taxonomy since about 1865. A special case of amyloidity is named hemiamyloidity. This peculiar colour reaction is so far only known in Ascomycetes, and of widespread occurrence, particularly in lichens but also in many Helotiales and some pyrenomycetes. The introduction of Melzer’s reagent into mycology since 1924 resulted in the failure to recognize the hemiamyloid reaction because of unfavourable properties of one of its ingredients: chloral hydrate.
Definition of hemiamyloidity. A microstructure reacts hemiamyloid when all the following apply: (1) direct application of Lugol’s solution (without KOH-pretreatment) provokes a red or red-brown reaction, (2) direct application of Melzer’s reagent yields no reaction at all (due to the high amount of chloral hydrate in that reagent), and (3) a blue reaction is obtained when the specimen is pretreated with KOH and then treated by either Lugol’s or Melzer’s. Euamyloidity means that the iodine reaction is blue with or without KOH-pretreatment, irrespective of which iodine reagent is applied. The superordinate name of these two variants is called amyloidity. A dextrinoid reaction displays a red or red-brown colour very similar to the hemiamyloid reaction, but differs in being entirely unchangeable by KOH, and in not being suppressed by chloral hydrate, but on the contrary often enhanced thereby.
A German version of this paper is published in the journal Tintling (Baral 2007), and a survey on this topic is found in Wikipedia (also in German):
http://de.wikipedia.org/wiki/Hemiamyloidität¤
http://de.wikipedia.org/wiki/Melzers_Reagenz
back to contents
Introduction
Among the chemical reagents used in mycology, iodine certainly constitutes the most important one. Already Nylander (1865) praised the iodine reaction of lichens as well as non-lichenizied ascomycetes as constant and very useful. Nevertheless, workers occasionally treat chemical characteristics with scepticism, tending to consider them less valuable than morphological characteristics. But which character is consistently present in every collection of a species which usually has it? In the case of iodine reactions variability actually occurs in some species. Usually it is, however, the method of the observer which leads to different results. It is the formula of the reagent as well as the loss of iodine concentration with the years, and in particular also the pretreatment with alkali which exert a crucial influence on the result (cfr. tabs. 1-2 and figs. 1-4).
For over 20 years I have been trying to convince ascomycete friends of the disadvantages of the currently applied Melzer’s reagent (briefly called Melzer’s or MLZ). Since about the middle of the last century this iodine solution has almost completely replaced Lugol´s solution (Lugol’s, IKI) which was in general use among mycologists in former times, whilst lichenologists never adopted Melzer’s reagent.
 Iodine crystals (I2) Iodine crystals (I2) |
 Potassium iodide crystals (KI) Potassium iodide crystals (KI) |
 Adding IKI …… Adding IKI …… |
 ….. to a preparation ….. to a preparation |
MLZ differs from IKI solely though seriously by the addition of ample chloral hydrate. IKI consists of 1 g iodine (I2) and 1-3 g potassium iodide (KI) (see fig. …) dissolved in 100-300 g water. MLZ contains about the same amounts of I2 and KI, but only 40-60 g water and further 40-60 g chloral hydrate. Why now this change to MLZ, and why only among mycologists? And what is so disadvantageous with MLZ, since it found such broad use nevertheless?
Actually, MLZ is a universal medium which combines the following advantages: (1) MLZ clears the preparation, thereby making complex structures more transparent since it quasi homogenizes the disturbing cell plasma (mainly because of a much higher refractive index compared to water); (2) MLZ kills living cells and thus makes preparations of fresh material compatible with preparations of herbarium material; (3) due to its high viscosity MLZ prevents annoying flow movements of spores, as well as the rapid drying of the preparation; and last but not least, (4) MLZ provides a valuable iodine reaction. It was the botanist A. Meyer (1907) who introduced the addition of chloral hydrate to IKI (under the name “Chloraljod”), in order to make tiny starch grains in chloroplasts more visible. Alternatively also lactic acid or lactophenol was added to the iodine solution. Melzer (1924) was obviously inspired by Meyer when he introduced his formula into mycology for the purpose of staining the ornamentation of Russula spores. My first attempts to reintroduce Lugol’s solution to mycology resulted in some disappointment because of the less clear microscopic image of an IKI preparation in comparison with MLZ.
back to contents
Hemiamyloidity
Where now are the disadvantages of MLZ? I discovered these quite early during my study of inoperculate discomycetes, simply because my “MLZ” oddly did not contain any chloral hydrate, and I did not know anything at all of the “high value” of that substance. Rather I was surprized that I repeatedly and constantly observed a red reaction in some species or genera, although in Dennis or elsewhere the obviously same species is said to be “I+ blue”, occasionally also “I-”. Much later I realized that some of the older authors, above all Rehm, observed just in these species not a blue but like me a red or “violet” reaction.
| inamyloid | hemiamyloid | euamyloid | ||||
| IKI | MLZ | IKI | MLZ | IKI | MLZ | |
| prior to KOH | ─ | ─ | red | ─ | blue | blue |
| after KOH | ─ | ─ | blue | blue | blue | blue |
| Tab. 1. Lugol’s (= IKI) allows a sharp separation between inamyloid, hemi- and euamyloid when applied to a non-pretreated preparation. On the contrary, Melzer’s (MLZ) requires pretreatment with KOH in the case of hemiamyloidity: this reagent gives a negative reaction when applied to a non-pretreated preparation. | ||||||
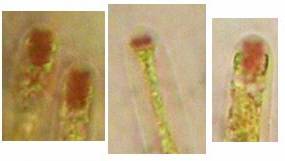
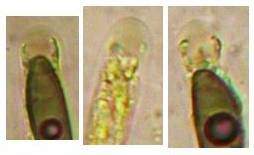
The high taxonomic value of this red reaction can hardly be overestimated. My current experience is that it is among the first key characteristics which comprise features like substrate, spore shape and size, guttules in the vital spores and paraphyses, croziers, and iodine reaction. If the latter is red, then often only a few or even a single species come into consideration in combination with the remaining data. In contrast to this, many detailed descriptions in the literature created from herbarium material are until today of unsettled identity, simply because the important characteristics are missing or unreliably reported, among other things concerning the iodine reaction. As an example, species have been described which are said to deviate from their near relatives merely by a missing iodine reaction (example: Velutarina rufoolivacea and V. juniperi). The “difference” lies here, however, not in the fungus but in the applied method (cfr. figs. 3-4).
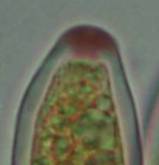
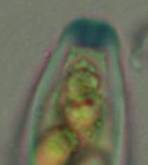
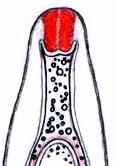
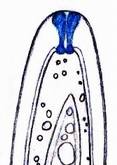
Concerning the red reaction of apical rings I more and more realized that I was dealing with a matter that has system and possesses validity within all Ascomycetes (I later termed it hemiamyloidity, Baral 1987). Two articles (Kohn & Korf 1975, Nannfeldt 1976) dealing with the serious influence of potassium hydroxide (KOH) on the iodine reaction helped me greatly in recognizing the general validity of the hemiamyloid reaction. According to these authors, KOH changes the chemical structure of the apical rings in such a manner that an intensively blue iodine reaction is obtained, where no color reaction was perceptible before. Of course both authors used MLZ (see figs. 3-4, right columns).
The guinea pigs of Kohn & Korf were mainly species of Pezicula. When I read that – and remembered that in Pezicula I had repeatedly seen a red-brown iodine reaction – I ordered commercial MLZ for the first time, and indeed: where I got a red reaction with IKI, MLZ showed almost no reaction! Only when I pre-treated with KOH, IKI as well as MLZ provoked an intense blue reaction. If I had at that time already read literature on lichens, then reports on a blue iodine reaction provoked by caustic solution surely would not have escaped my notice. The conversion from red to blue by means of KOH (figs. 1-2) was well-known among lichenologists already for one hundred years, but this realization never penetrated into mycology. Thus the scapegoat was found: chloral hydrate (like, by the way, other different viscose additives) suppresses the red reaction! In the consequence I tested many dozens of Helotiales species with red-reacting apical rings, and KOH-treatment always caused these to react blue in iodine.
My later studies revealed that Pezicula is one of the few genera whose representatives all react consistently, intensively, and purely red with Lugol’s. Hence, Kohn & Korf discovered the necessity for the KOH pretreatment in this genus, and generally recommended this pretreatment in Ascomycetes, in order not to speak misleadingly of iodine-negative asci. The use of KOH as a preparation medium is a general method in herbarium mycology for swelling the dry fungus fragment on the slide prior to microscopy. The iodine reaction was therefore frequently tested after swelling in KOH, particularly after Kohn & Korf’s paper was published, though not consistently. However, even since, data on whether KOH was applied is not infrequently lacking in the literature.
After Kohn & Korf’s observation became public, KOH frequently appeared in association with MLZ in the descriptions of authors. But only if authors had implemented the iodine test both before and after KOH treatment, the use of MLZ and KOH permits the distinction of three instead of (as before) two possibilities: (1) inamyloid, (2) hemiamyloid (= KOH-provoked amyloidity), (3) euamyloid. Now I was able to indirectly conclude that a red IKI reaction would occur in a fungus in which the MLZ test without KOH was reported as negative, whilst that after KOH positive.
The reader may already suspect that I am unsatisfied with the often seen literature reports stating the iodine reaction briefly as “J+” or “J–”. Apart from the shape of the apical rings being quite different from genus to genus, and the intensity of the reaction, the color as well as the method must be exactly described. If one nevertheless wants to insist on the usage of MLZ, then an iodine test is to be made prior to and after KOH treatment. Why I remained faithful for my beloved Lugol’s solution, has, apart from the striking feature of the red reaction, different reasons. On the one hand the KOH pretreatment is rather time-consuming, while the IKI test is very fast. Further, often the case is that the reaction is blue with low iodine concentration, but changes to red with higher concentration. If the entire ascus wall is reactive, then the hymenia stain like a rainbow in this case (fig. 5). When using MLZ one does not recognize this special case of hemiamyloidity, which is obviously based on the mixed presence of red and blue reacting substances. It is particularly common with lichens in general, and now we understand also, why lichenologists continued to use Lugol’s all the time. They use, by the way, more strongly diluted Lugol ‘s solution (urine yellow instead of red-brown), because with lichens the entire ascus wall reacts intensely (sometimes even the gel around the paraphyses), so that the important apical structures can only be studied at a low iodine concentration. At such low concentration one does not always obtain the color change from blue to red, however.
|
IKI-reaction (without KOH) |
euamyloid | hemiamyloid | |
| Type BB | Type RB | Type RR | |
| high iodine concentration | blue | dirty red | pure red |
| low iodine concentration | blue | blue | pure red |
| Tab. 2. Three types of amyloidity can be distinguished when applying IKI to a fungus not pretreated with KOH: RB is a type intermediate between BB and RR, but is subsumed under hemiamyloid because of the presence of the red IKI reaction which is converted to blue when KOH-pretreated. | |||
In order to give names to the three cases of amyloidity, I introduced the abbreviations BB, RB and RR. In the case of RB one could speak also of intermediate or mixed hemiamyloidity. Actually there exist all transitions between BB and RR, in a way that the critical iodine concentration, at which the color change takes place, lies in one case at approximately 0.05% iodine, in the other more around 0.5%. The higher this critical concentration, the more dirty is the colour of the red reaction. Thereby the red colour is infered with the blue, and a rather dirty red reaction is caused by a relatively high amount of euamyloid substance. Because of these continuous transitions I like to use also the abbreviations Rb (and rB), in order to refer to reactions in which the blue (or the red) arises only within a very narrow concentration range and thus will be easily overlooked. Of course these are no sharply circumscribed categories, on the contrary there seems to exist every mixing proportion among the species. This mixing proportion may, however, vary to some degree within a certain species, depending on the collection. That a color change takes place at all originates from the fact that the blue reacting (euamyloid) substance has a higher affinity to iodine, and therefore gets stained at a considerably lower iodine concentration (about 0.01-0.1%) in comparison to the red reacting (hemiamyloid) substance, which is recognizable only above ca. 0.03-0.2% iodine. It was in fact 140 years earlier when the lichenologist Füisting (1868) proposed the idea that in hymenia of lichens blue- and red-reacting substances might coexist in varying proportions.
Some MLZ proponents obtain the red reaction with MLZ, but only at short notice, in that they let MLZ diffuse laterally into the water mount (as I also do with IKI, see fig. …). That works because iodine draws in faster than the slower chloral hydrate. Finally, IKI permits vital staining, MLZ not. With IKI the cell nucleus and various other plasmatic structures become more clearly visible, and that exclusively in the living cell. The fact that the apical rings of the asci swell strongly in height if the asci are killed, escaped notice of herbarium mycologists because they tend to prepare their fresh finds, for sake of convenience, straight in the lethal MLZ (or in lactophenol or KOH). However, living cells do not require any mountants for clearing or swelling, on the contrary: the high-contrast microscopic image is substantially deteriorated with the addition of lethal media such as MLZ or KOH.
back to contents
Chemical background
The blue color induced by iodine solutions is generally attributed to the storage of the iodine atoms into screw-shaped carbohydrate macromolecules, for instance in the case of starch (reserve substance of plants). Thereby the length of the threadlike molecule is said to affect the color: blue if long, red if short. The related reserve substance of animals, glycogen, likewise forms helicoid molecules and is said to react red because of its many branchings. In ascus walls similar helix-shaped macromolecules might be responsible for their amyloidity, and this all the more, as only such screw-shaped microstructures enable the observed enormous elasticity of the apical rings. Likewise, the amyloidity of the highly elastic lateral wall of the sac-shaped asci of the Lecanorales would herewith be explained. Which substance is responsible for ascus amyloidity is not clarified until today, however. My earlier theory that it could be the amyloid lichen substance “Isolichenin”, was disproved by Common (1991): according to this author there are salient differences in the reaction behavior between the sterile parts of lichens (thallus, excipulum, paraphyses) and the fertile parts (asci, ascogenous hyphae): like the fertile parts, the sterile parts react either blue (“Isolichenan”) or red (“Lichenan”); if KOH-pretreated, however, the amyloid substance of the sterile parts loses some of its reaction intensity, while no change in color is obtained, contrary to the amyloid substance of the fertile parts which Common calls “Amylomycan”. In addition, when staining (Iso)Lichenan the iodine reaction does not fade and disappear with heating up the preparation, while Amylomycan completely decolorizes rapidly and reversibly thereby. Among the lichenized Ascomycetes, the Arthoniales form an exception, in which also the sterile tissue contains Amylomycan.
Finally I wish to mention that I tried to explain the chemistry of the red hemiamyloid reaction by the hypothesis that treatment with KOH induces a stronger spiral rolling up of the threadlike macromolecules, resulting in a change of the iodine reaction from red to blue. The simultaneous occurrence of blue and red colours in unpretreated ascus walls would easily be explained by a mixed presence of spirals with different strength of spiral rolling. The chemical change of the spirals provoked by KOH is irreversible – once treated with KOH the red reaction (hemiamyloidity) is not detectable any longer. One can obtain this effect of rolling up also by cooking in water for 10-15 min, while even strong acids do not induce any such effect. Also long storage in the herbarium over several decades occasionally provokes loss of hemiamyloidity. E.g., voucher specimens of Pezicula react blue instead of red in IKI if older than about 40-50 years while various other Helotiales still react red when older than 100 years. This is the reason why Verkley (1999) in his monograph on Pezicula reported a blue IKI reaction in “most of the herbarium material”, and therefore somewhat doubted the value of the hemiamyloid reaction. Why he misleadingly noted the reaction in several species to be variable (“IKI+ or -“), weakly red or blue, or negative (“IKI-“), can perhaps be explained by a much too low-concentrated IKI solution.
back to contents
Dextrinoidity
In the case of the so-called dextrinoid reaction Melzer’s is superior to Lugol’s. This type of red or brown-red reaction is particularly to be seen in various Basidiomycetes (examples: gill trama of Mycena, spore walls of Lepiotaceae), which explains why MLZ was first introduced by basidiomycetologists. The core of the matter is that the chloral hydrate does not suppress the red reaction here, but even strengthens it, and occasionally provokes the reaction. Moreover, not even the most intense KOH-pretreatment induces a blue iodine reaction here, but occasionally does result in a reinforcement of the red reaction. For this reason, basidiomycetologists have good reason to continue employing Melzer’s reagent (see Leonard 2006).
|
|
|

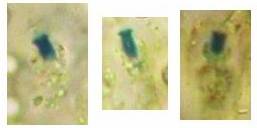
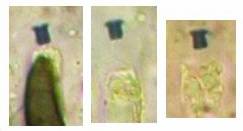
Fig. 13. Orbilia ovoidea, dead asci in IKI, ascoplasma stained red because of glycogen. |
|
| Fig. 11. Ascospores of Lachnellula angustispora, dead state, in IKI, with two large glycogen areas which are surrounded by small oil drops. | Fig. 12. Conidium of Monacrosporium psychrophilum (anamorph of Orbilia aff. auricolor), vital state, in IKI, with large glycogen areas. |
A completely different cell wall composition must be the basis of dextrinoidity (in basidiospores due presumably to a glycine-betaine complex, see Leonard l.c.). A dextrinoid reaction very rarely occurs in ascus walls (apical thickening in some Orbiliaceae), while the hemiamyloid reaction is rarely to be observed elsewhere than in ascus walls, and is so far completely unknown in Basidiomycetes. As an example, dextrinoid reactions occur in the hairs and excipular cells of some Hyaloscypha taxa. Their copper-reddish reaction in MLZ is used in Huhtinen´s (1990) monograph as species-characteristic, though not always very clear and apparently also somewhat variable. However, when applying IKI to unpretreated preparations of Hyaloscypha this dextrinoid reaction is imposible to observe. Also the iodine reaction of glycogen can be classified as dextrinoid (figs. 11-13): here chloral hydrate or KOH provoke neither a reinforcement nor a weakening of the red reaction. This reserve substance (carbohydrate), being widespread in fungal cells, occurs particularly in submature asci, but also in mature spores. In asci the presence of glycogen is occasionally misinterpreted as a hemiamyloid reaction; however, glycogen may easily be differentiated by its situation within the ascoplasma as opposed to the apical rings located within the ascus wall, and, of course, by the impossibility of being converted to a blue reaction by KOH.
back to contents
Hints on practical application
Lugol’s and Melzer’s can very easily be manufactured, thereby it is not necessary to weigh out the ingredients. Since iodine is very volatile one can keep rather stable concentrations only in great quantities (100-200 ml) within large bottles. It is important that the solution in a 10 ml bottle looks dark red-brown (fig. 14), which corresponds very roughly to a one percent iodine concentration. A few blackish iodine crystals and about the three-fold quantity of white potassium iodide crystals are given into 10 ml distilled or tap water. After a few shakes and leaving the whole untouched one day at room temperature, everything will be dissolved. Glass bottles with glass pipette are better than plastic which is not a barrier for iodine. If the colour of the solution fades somewhat within perhaps a year, further iodine crystals may be added. The KOH-pretreatment requires a roughly 2 to 5 percent KOH solution. NaOH solution does the same service. The induction period lasts a few seconds up to approximately one minute, while a longer influence does no harm. In some tough cases a flame can be held under the slide for some seconds which accelerates the process (obligatory with hemiamyloid excipular tissues, figs. 9-10). Generally I add a drop of KOH to the edge of the cover glass of an IKI preparation and let it draw in and discolour the iodine. After about half a minute I add plenty of IKI to the KOH mount. Since KOH decolorizes IKI by transforming iodine (I2) into potassium iodide (KI) and therefore destroying its reactivity, one has to take off the surplus KOH by means of filter paper, before adding iodine.
| Fig. 14. IKI with optimum iodine concentration of roughly 1% |
Since KOH is a widespread and useful medium for the investigation of herbarium material, I once more wish to draw attention to the necessity to perform the IKI test prior to swelling in KOH in order to test for hemiamyloidity. Generally, one should add only a small drop of Lugol’s to the preparation, in order to be able to differentiate between RR and RB, i.e., to recognize whether a colour change from blue to red takes place. Particularly if only having small fragments under the cover glass, the iodine concentration rapidly exceeds the critical value. It is therefore favourable to squeeze larger apothecial fragments prior to adding iodine. Then most of the iodine gets caught at the periphery and a continuous gradient of iodine concentration towards the centre is obtained. One can thus get a permanently blue reaction in the centre while the red reaction is only seen at the periphery.
Those who want to test hemiamyloidity should be aware of the fact that there are many species with a weak or dirty red reaction of their apical rings, which are not easily recognized, even under oil immersion, especially if the rings are very small. A recommendable discomycete for beginners, though not easily to be found, is the genus Pezicula with its large asci. Also the frequent Lachnellula occidentalis is very suitable, although here inamyloid asci occur with varying frequency. Unfortunately, the majority of the more frequent Helotiales species react blue or only dirty red. Beautiful red reactions are exhibited, for instance, in species of Allophylaria and in the Hysteropezizella complex (including Coronellaria and Cejpia). To collect lichen apothecia is very easy, in which, as also in many Ostropales, the whole hymenium virtually always reacts hemiamyloid. Among the pyrenomycetes almost exclusively Nemania serpens and species of Hypocopra have red-reacting apical rings. In addition the hymenial gelatine of Capronia and Epibryon stain mostly distinctly red. Within the Pezizales I so far only know a hemiamyloid reaction in Pachyella and Iodophanus.
I am very thankful to Richard P. Korf for his linguistic suggestions, and to Thomas Harbich for technical help.
back to contents
References
BARAL, H.O. (1987). Lugol’s solution/IKI versus Melzer’s reagent: hemiamyloidity, a universal feature of the ascus wall. Mycotaxon 29 : 399‑450.
— (2007). Über die Jodreaktion bei Ascomyceten. Tintling 12(2): 16-22.
COMMON, R. (1991). The distribution and taxonomic significance of lichenan and isolichenan in the Parmeliaceae (lichenized Ascomycotina), as determined by iodine reactions. I. Introduction and methods, II. The genus Alectoria and associated taxa. Mycotaxon 41 (1) : 67‑112
FÜISTING, W. (1868). Beiträge zur Entwicklungsgeschichte der Lichenen 2: Entwicklung der Perithecien des Endopyrenium monstruosum, pusillum und Endocarpon miniatum. Botanische Zeitung 26: 657-663.
HUHTINEN, S. (1990). A monograph of Hyaloscypha and allied genera. Karstenia 29 (2): 45-252.
KOHN, L.M.; R.P. KORF (1975). Variation in ascomycete iodine reaction: KOH pretreatment explored. Mycotaxon 3: 165-172.
LEONARD, L.M. (2006). Melzer’s, Lugol’s or iodine for identification of white-spored Agaricales? McIlvainea 16(1): 43-51.
MELZER, M.V. (1924). L’ornementation des spores de Russules. Bull. Soc. myc. Fr. 40: 78-81.
MEYER, A. (1907). Erstes mikroskopisches Praktikum. Eine Einführung in den Gebrauch des Mikroskops und in die Anatomie der höheren Pflanzen. 2. ed. Fischer, Jena.
NANNFELDT, J.A. (1976). Iodine reactions in ascus plugs and their taxonomic significance. Trans. Brit. Mycol. Soc. 67 (2): 283-287.
NYLANDER, W. (1865). Ad historiam reactionis iodi apud Lichenes et Fungos notula. Flora (Allg. Bot. Zeitung, Regensburg) 48 (30): 465-468.
VERKLEY, G. (1999). A monograph of the genus Pezicula and its anamorphs. Stud. Mycol. 44: 1-180.